- A company that meets the needs of people around the world through superior products and services
Products List

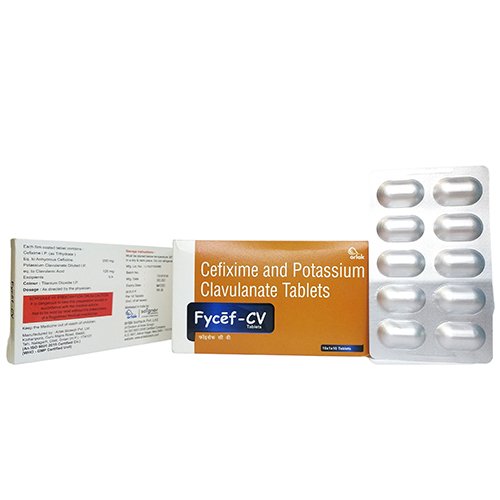
Fycef-CV is the combination of Cefixime & Potassium Clavulanate Tablets that are used to treat some infections caused by bacteria. These include infections of lungs, ear, respiratory tract infection, urinary tract infection, bone, skin and sinus. Cefixime belongs to a class of drugs known as antibiotics and Clavulanate to beta-lactamase inhibitors. Cefixime & Potassium Clavulanate Tablets help to stop the formation of a bacterial protective layer that is necessary for the survival of the bacteria and hence it stops the infection from spreading.
Precautions for Cefixime & Potassium Clavulanate Tablets
Cefixime & Potassium Clavulanate Tablets should be taken after eating a meal and with it plenty of water must be consumed to get protected from the possible side effects. None of the doses must be skipped and the doctor must be consulted if the patient is pregnant.
Side Effects of Cefixime & Potassium Clavulanate Tablets
Cefixime & Potassium Clavulanate Tablets may come up with some side effects which can’t be ignored and the doctor must be consulted if the situation doesn’t get better with time.
- Diarrhea
- Nausea
- Stomach Pain
- Indigestion
- Flatulence
Contact for Cefixime & Potassium Clavulanate Tablets Manufacturing And Supply
Soigner Pharma is one of the best names in the Pharmaceutical sector nowadays. The company has ISO certification and works for the well-being of mankind and their medical needs. We are open to serve our associates through our services and are committed to providing them with the best quality pharma products. We are also acknowledged as the best Cefixime and Potassium Clavulanate Tablets manufacturer and supplier in India. The quality of the products is never sacrificed by us. Soigner Pharma also offers PCD Pharma Franchise for Cefixime and Potassium Clavulanate Tablets with many benefits.
- We have a vast range of DCGI and FSSAI approved pharma products available at a very nominal rate.
- The logistics services of our company ensure the on-time delivery of the products that we offer.
- Our skilled production team manufactures the products in accordance with the norms of WHO and GMP.
- The company has a well-qualified R & D that brings the latest medicines to the market after doing so much research.
| Brand Name | Fycef-CV |
|---|---|
| Composition | Cefixime 200 mg & Potassium Clavulanate 125 mg |
| Pack Type | ALU-ALU |
| Pack Size | 1 X10 |

 Get a Quote
Get a Quote