- A company that meets the needs of people around the world through superior products and services
Products List

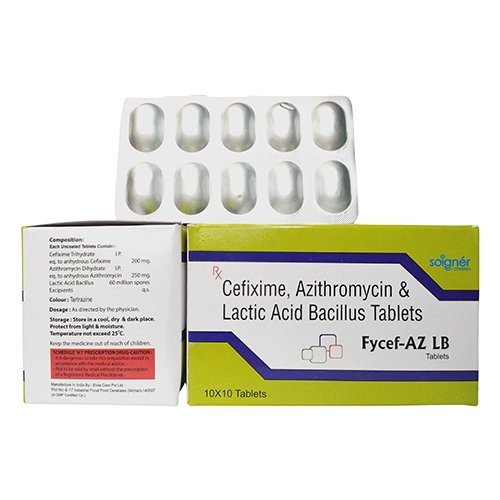
FYCEF-AZ LB is the combination of three formulations: CEFIXIME, AZITHROMYCIN & LACTIC ACID BACILLUS TABLETS.
Cefixime, an antibiotic, is a third-generation cephalosporin like ceftriaxone and cefotaxime. Cefixime is highly stable in the presence of beta-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins due to the presence of beta-lactamases, may be susceptible to cefixime. The antibacterial effect of cefixime results from inhibition of mucopeptide synthesis in the bacterial cell wall.
Azithromycin is a semi-synthetic macrolide antibiotic of the azalide class. Like other macrolide antibiotics, azithromycin inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit of the bacterial 70S ribosome. Binding inhibits peptidyl transferase activity and interferes with amino acid translocation during the process of translation. Cefixime, Azithromycin and Lactic Acid Bacillus Tablet’s effects may be bacteriostatic or bactericidal depending of the organism and the drug concentration. Its long half life, which enables once daily dosing and shorter administration durations, is a property distinct from other macrolides.
Azithromycin, a semisynthetic antibiotic belonging to the macrolide subgroup of azalides, is used to treat STDs due to chlamydia and gonorrhea, community-acquired pneumonia, pelvic inflammatory disease, pediatric otitis media and pharyngitis, and Mycobacterium avium complex (MAC) in patients with advanced HIV disease. Similar in structure to erythromycin. Cefixime, Azithromycin and Lactic Acid Bacillus Tablets zithromycin reaches higher intracellular concentrations than erythromycin, increasing its efficacy and duration of action.
PHARMACOKINETICS: –
CEFIXIME: –
Absorption: – About 40%-50% absorbed orally whether administered with or without food, however, time to maximal absorption is increased approximately 0.8 hours when administered with food. Protein binding: – 65% (concentration-independent)
Metabolism: – Hepatic. Approximately 50% of the absorbed dose is excreted unchanged in the urine in 24 hours.
Half life : – 3-4 hours (may range up to 9 hours). In severe renal impairment (5 to 20 mL/min creatinine clearance), the half-life increased to an average of 11.5 hours.
AZITHROMYCIN: –
Absorption: -Bioavailability is 37% following oral administration. Absorption is not affected by food. Azithromycin is extensively distributed in tissues with tissue concentrations reaching up to 50 times greater than plasma concentrations. Drug becomes concentrated within macrophages and polymorphonucleocytes giving it good activity against Chlamydia trachomatis.
Volume of distribution:-31.1 L/kg
Protein Binding: -Serum protein binding is variable in the concentration range approximating human exposure, decreasing from 51% at 0.02 µg/mL to 7% at 2 µg/mL.
Metabolism : -Hepatic. In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed.
Route of elimination: – Biliary excretion of azithromycin, predominantly as unchanged drug, is a major route of elimination.
Half life: -68 hours
Clearance : –apparent plasma cl=630 mL/min [following single 500 mg oral and i.v. doses]
INDICATION For use in the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
(1) uncomplicated urinary tract infections caused by Escherichia coli and Proteus mirabilis,
(2) otitis media caused by Haemophilus influenzae (beta-lactamase positive and negative strains), Moraxella catarrhalis (most of which are beta-lactamase positive), and S. pyogenes,
(3) pharyngitis and tonsillitis caused by S. pyogenes,
(4) acute bronchitis and acute exacerbations of chronic bronchitis caused by Streptococcus pneumoniae and Haemophilus influenzae (beta-lactamase positive and negative strains), and
(5) uncomplicated gonorrhea (cervical/urethral) caused by Neisseria gonorrhoeae (penicillinase- and non-penicillinase-producing strains).
FYCEF-AZ consists of tablets that are a fusion of three medicines: Cefixime, Azithromycin, and Lactobacillus. These tablets are used to fight with different types of bacterial infections.
It fights against the microorganisms to prevent their growth and the further spread of the infection. It also prevents diarrhea which may occur as a side effect of this medicine.These Tablets belong to the third generation cephalosporin antibiotics.
Precautions for Cefixime, Azithromycin, and Lactobacillus Tablets
Cefixime, Azithromycin, and Lactobacillus tablets may be taken with or without food but it must be taken at a fixed time to ensure better efficacy. The patient should avoid consuming alcohol. Plus the patient should tell the doctor if suffering from a liver or kidney disease. Take a healthy diet and drink plenty of water.
Side effects of Cefixime, Azithromycin, and Lactobacillus Tablets
Cefixime, Azithromycin, and Lactobacillus tablets may cause some side effects which don’t require any medical attention but if they persist then let the doctor know about them right away.
- Nausea
- Vomiting
- Headache
- Loss of appetite
- Flatulence
- Diarrhea
- Stomach Pain
Contact for Cefixime, Azithromycin, and Lactobacillus Tablets Manufacturing And Supply
Soigner Pharma is a Well-reputed company in the Indian pharma sector. We are an ISO certified company and we deal in quality pharma products at very fair rates. Soigner Pharma is also a third party Cefixime, Azithromycin, and Lactobacillus tablets manufacturer and supplier in India. We have well experienced staff that have been serving mankind for so many years. We also offer PCD Pharma Franchise for Cefixime, Azithromycin, and Lactobacillus tablets with complete monopoly rights.
- Our company offers a broad range of FSSAI and DCGI approved pharma products.
- Our Manufacturing plant is well equipped with hi-tech machinery and a very clean environment.
- Our R & D team constantly works on new molecules and delivers new products to the market.
- Our company delivers the best quality products in very less time
| Brand Name | FYCEF-AZ LB |
|---|---|
| Composition | CEFIXIME 200 mg + AZITHROMYCIN 250 mg + LACTIC ACID BACILLUS 60 MILLION SPORES |
| Pack Type | ALU-ALU |
| Pack Size | 10X1X10 |

 Get a Quote
Get a Quote