- A company that meets the needs of people around the world through superior products and services
Products List

Meropenem Injection is a broad-spectrum carbapenem antibiotic. It is active against Gram-positive and Gram-negative bacteria. Meropenem exerts its action by penetrating bacterial cells readily and interfering with the synthesis of vital cell wall components, which leads to cell death.
PHARMACOLOGY
PHARMACODYNAMICS: – The bactericidal activity of meropenem results from the inhibition of cell wall synthesis. Meropenem readily penetrates the cell wall of most Gram-positive and Gram-negative bacteria to reach penicillin-binding- protein (PBP) targets. Its strongest affinities are toward PBPs 2, 3, and 4 of Escherichia coli and Pseudomonas aeruginosa; and PBPs 1, 2, and 4 of Staphylococcus aureus.
PHARMACOKINETICS: –
• ROUTE OF ELIMINATION: – Approximately 70% of the intravenously administered dose is recovered as unchanged meropenem in the urine over 12 hours, after which little further urinary excretion is detectable.
• HALF-LIFE: – Approximately 1 hour in adults and children 2 years of age and older with normal renal function. Approximately 1.5 hours in children 3 months to 2 years of age.
INDICATIONS: – For use as single-agent therapy for the treatment of the following infections when caused by susceptible isolates of the designated microorganisms: complicated skin and skin structure infections due to Staphylococcus aureus (b-lactamase and non-b-lactamase producing, methicillin-susceptible isolates only), Streptococcus pyogenes, Streptococcus agalactiae, viridans group streptococci, Enterococcus faecalis (excluding vancomycin-resistant isolates), Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Bacteroides fragilis, and Peptostreptococcus species; complicated appendicitis and peritonitis caused by viridans group streptococci, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacteroides fragilis, B. thetaiotaomicron, and Peptostreptococcus species. Also for use in the treatment of bacterial meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae (b-lactamase and non-b-lactamase-producing isolates), and Neisseria meningitis.
Directions For Use
As the medication is available in the form of injection, so the person is advised to get the injection instilled in his vein or the muscle by the doctor or the nurse. The person should visit the hospital to get the medication.
Side effects Of Meropenem Injection
Depending on the body of the person, the following are the side effects that may or may not occur after the instilling of the injection :
- Pain
- Nausea
- Vomiting
- Headache
- Constipation
Precautions To Be Taken
The precautions which are necessary to consider are as follows :
- The person participating in regular physical activity is advised not to undergo the treatment of this injection.
- If the person experiences watery and bloody stools then he should consult the doctor immediately.
- The person suffering from the brain tumor is advised to consult the doctor as there is a possibility of interaction of both the medications.
- The consumption of alcohol is not considered safe during the treatment.
Frequently Asked Questions (FAQs)
- What special advice is to be followed during the treatment of Meropenem Injection?
To avoid the interaction, the person is advised not to take the valproic acid during the treatment. The person is advised to monitor his kidney functions as well as the blood count on the regular basis.
- Can the person suffer from allergic reactions after the use of the medication?
Yes, the person may suffer from allergic reactions. If the person suffers from itching or swelling in the hands or face, then he is advised to consult the doctor.
| Brand Name | SIGMERO-1000 |
|---|---|
| Composition | Meropenem 1000 mg |
| Pack Type | VIAL |

 Get a Quote
Get a Quote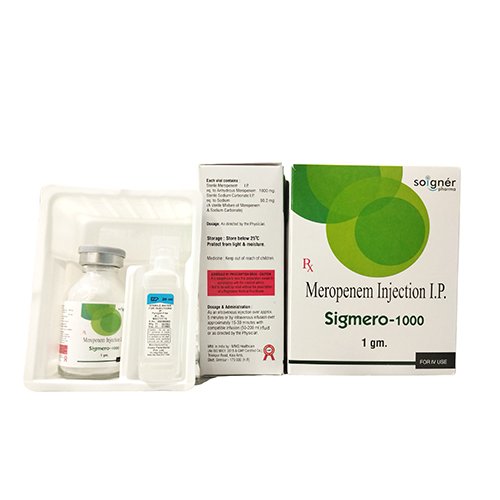



Reviews
There are no reviews yet.