- A company that meets the needs of people around the world through superior products and services
Products List

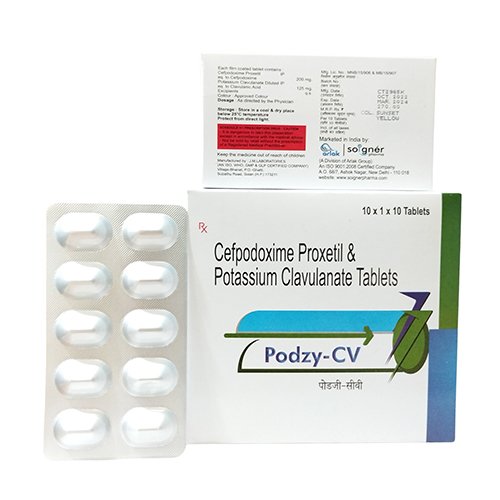
PODZY CV is a fusion of two medicines ie. Cefpodoxime and Clavulanic Acid Tablets. These tablets are used to treat respiratory tract infections. Respiratory tract infections are the infections that affect the sinuses, throat, airways or lungs. Cefpodoxime and Clavulanic Acid Tablets fights against the infection by stopping its growth and further spread of the same. These Tablets belong to the third-generation cephalosporin antibiotics.
Precautions for Cefpodoxime and Clavulanic Acid Tablets
Cefpodoxime and Clavulanic Acid tablets can be taken with or without food but it must be taken at a fixed time to ensure its better efficacy. The patient should avoid consuming alcohol. Let the doctor know if the patient is having a liver or kidney disease. It is safe to take these tablets during pregnancy. Take a healthy diet and drink plenty of water to avoid dehydration.
Side effects of Cefpodoxime and Clavulanic Acid Tablets
Cefpodoxime and Clavulanic Acid tablets may cause some side effects which don’t require any medical attention but if they don’t go away with time then let the doctor know about them right away.
- Nausea
- Vomiting
- Diarrhea
- Indigestion
- Stomach Pain
- Allergic Reaction
Contact for Cefpodoxime and Clavulanic Acid Tablets Manufacturing And Supply
Soigner Pharma is a Well-reputed company in the pharmaceutical sector. We are an ISO certified company and we deal in quality pharma products at very nominal rates. Soigner Pharma is a well renonwned third party Cefpodoxime and Clavulanic Acid tablets manufacturer and supplier in India. We have well experienced staff that have been serving mankind for so many years. Our firm also offer PCD Pharma Franchise for Cefpodoxime and Clavulanic Acid tablets with complete monopoly rights.
- Our company offers a broad range of DCGI and FSSAI approved pharma products.
- Our Manufacturing plant is well equipped with world class machinery.
- Our R & D team constantly works on new products and delivers them to the market.
- Our company always delivers the best quality products in very less time.
More Information
Cefpodoxime and Clavulanic acid combination are effective against multiple infections. The pharmacological action of Clavulanic acid prevents hydrolysis of Cefpodoxime against beta-lactamase secreting microbes and increases the antibiotic spectrum. Cefpodoxime, an antibiotic belongs to class of third-generation cephalosporins, is indicated for the treatment of systemic infections including respiratory tract infections, urinary tract infections, otitis media, skin infections and uncomplicated gonorrhoea due to Gram-positive and Gram-negative organisms. A Combination of Cefpodoxime (third generation oral cephalosporin) and a beta lactamase inhibitor, Clavulanic Acid, used for the treatment of urinary tract infections, pneumonia, bronchitis, gonorrhea, and multiple other infections.
Pharmacology
Pharmacodynamics: – Cefpodoxime, a third generation semi-synthetic cephalosporin, exhibits activity against several Gram positive as well as Gram negative microorganisms. This compound is also stable in beta lactamase environment. Cefpodoxime exhibits exceptional activity against methicillin susceptible Staphylococci, Streptococcus pneumoniae, Haemophilus influenzae, Nesseria spp, and Moxaxella catarrhalis, which are referred as the most common hospital acquired and community acquired infections. Clavulanic acid is a natural inhibitor of beta lactamase, which are produced by Streptomyces clavuligerus. It binds to beta lactamase moieties and inactivates them, thus restricting the cefpodoxime destruction. Clavulanic acid has very little antimicrobial activity.
Pharmacokinetics: – Cefpodoxime Bioavailability of cefpodoxime is 50% in fasting subjects and it increases in presence of food. Peak plasma concentration of Cefpodoxime 200 mg single dose is 2.18 mcg/ml. The Drug is well distributed after oral administration. Cefpodoxime reaches therapeutic concentrations in respiratory tract and genito-urinary tracts and bile. Protein binding of cefpodoxime ranges from 20 to 30 %. The plasma half life of cefpodoxime is about 2 to 3 hours and is prolonged in patients with impaired renal function. Cefpodoxime is excreted unchanged in urine. Clavulanic Acid Clavulanic acid is well absorbed after oral administration. Peak plasma concentration of Clavulanic acid 125 mg single dose is 2.2 mcg/ml. It is distributed completely after oral administration.
Protein binding of clavulanic acid is about 30 %.The plasma half life of clavulanic acid is one hour. About 60 % of clavulanic acid is excreted unchanged in urine. The clavulanic acid component in the drug combination protects cefpodoxime from degradation by ß-lactamase enzymes, and effectively extends the antibiotic spectrum of cefpodoxime to include many bacteria that are normally resistant to cefpodoxime and other ß-lactam antibiotics. Indications Acute bacterial exacerbations of chronic bronchitis Acute community acquired Pneumonia Upper and lower respiratory tract infections Skin and soft tissue infections Urinary tract infections Pharyngitis and/or tonsillitis General gonorrhea (men and women) and rectal gonococcal infections (women) Acute maxillary sinusitis.
| Brand Name | PODZY CV |
|---|---|
| Composition | CEFPODOXIME 200 MG + CLAVUNANIC ACID 125 MG |
| Pack Type | ALU-ALU |
| Pack Size | 1X10 |

 Get a Quote
Get a Quote